- Show Menu
- Contact Us
- FAQs
- Reader Service
- Survey Data
- Survey Winners
- Testimonials
- Upcoming Events
- Webinars
New & Noteworthy
August/September 2024
Rapid Membrane Enzyme Immunoassay
TECHLAB, Inc
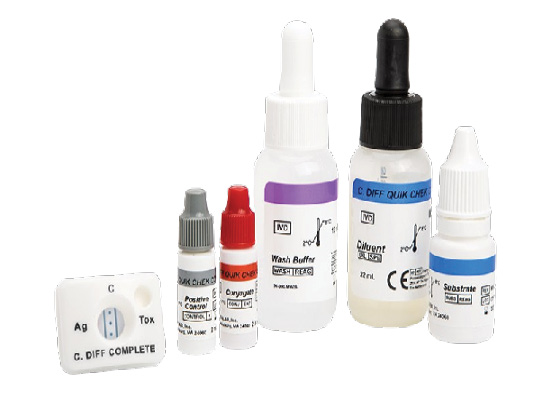
TECHLAB, part of the SSI Diagnostica Group, announces that its C. DIFF QUIK CHEK COMPLETE test, a rapid membrane enzyme immunoassay designed for the simultaneous detection of Clostridioides difficile GDH antigen and toxins A and B in a single test, has received certification under the European In Vitro Diagnostic Medical Device Regulation (IVDR). The test screens for the presence of C. diff, detects C. diff antigen and toxin, and confirms the presence of toxins A and/or B.
Recent Popular Articles
About Us
MedicalLab Management Ridgewood Medical Media, LLC
Quick Links
Subscribe to Our Email Newsletter!
© 2005 - 2025 MLM Magazine - MedicalLab Management.
All rights reserved.